In the summer of 2010, bermudagrass (Cynodon dactylon (L.) Pers.) hay producers in Georgia counties (JeffDavis, Irwin, Pierce, and Tift) began noticing a “bronzing” of their hay fields, generating damage similar to that of severe drought- or frost-damaged bermudagrass (Figure 1A). The bronzing was the result of chlorosis and necrosis in the top two to three leaves of the plant (Figure 1B). The damaged leaves could easily be pulled fromthe sheath, and the end inside the sheath either showed evidence of insect damage or obvious decay (Figure 1C).The collected larvae were grown out and allowed to pupate and mature. The resulting adults were subsequently identified as Atherigona reversura Villeneuve (Diptera: Muscidae), now commonly known as the bermudagrass stem maggot (BSM).
 Figure 1A. “Bronzing” of bermudagrass hay fields as a result of bermudagrass stem maggot damage. Photo by Will Hudson
Figure 1A. “Bronzing” of bermudagrass hay fields as a result of bermudagrass stem maggot damage. Photo by Will Hudson
 Figure 1B. The bronzing is the result of damage done at the uppermost node that results in the deterioration of the top two to three leaves of the plant. Photo by Lisa Baxter.
Figure 1B. The bronzing is the result of damage done at the uppermost node that results in the deterioration of the top two to three leaves of the plant. Photo by Lisa Baxter.
 Figure 1C. The damaged leaves can easily be pulled from the sheath and the end inside the sheath shows evidence of insect damage or obvious decay. Photo by Lisa Baxter.
Figure 1C. The damaged leaves can easily be pulled from the sheath and the end inside the sheath shows evidence of insect damage or obvious decay. Photo by Lisa Baxter.
The BSM is believed to be native to Southeast Asia, which is where it was first discovered. Since the 2010 discovery in southern Georgia, the BSM has spread throughout the Southeast, damaging bermudagrass turfgrass, hayfields, and pastures as far north as North Carolina and Kentucky and as far west as Texas.
Yield Losses
In general, each Atherigona species has its own preferred host. Though it may be found on or around other grass species, A. reversura has only been found to damage bermudagrass and stargrass (C. nlemfuensis) in the United States.
The larva of BSM bores into the pseudostem, the stem-like structure made up of leaf sheaths, where it macerates the vascular tissue. It feeds on the sap and microbial soup that it creates from the macerated tissue. This feeding occurs outward from the last node of the plant, which cuts off water and sap flow to and from the top two to three leaves. The amount of yield loss caused by this feeding depends upon the stage of growth wherein the damage occurs.
If the damage occurs once the bermudagrass is nearing harvest, the loss of those top two to three leaves may reduce the yield by less than 10% for that cutting. However, if the damage occurs during the early stages of regrowth, affecting less than 6 inches (15 centimeters) of new growth, yield losses can be severe. Yield losses in excess of 80% have been reported in bermudagrass hayfields in the latter part of the season.
Since bermudagrass is grown for hay and pasture—around 300,000 and 3 million acres, respectively, in Georgia alone—on so many acres across the Southeast, the economic impact of the BSM is substantial. Conservatively, a total yield loss of up to 3 tons/acre (6,700 kg/ha) could be expected in a typical bermudagrass hayfield in south Georgia if the BSM was left untreated. Depending on the quality and market for this forage, a loss of 3 tons/acre could represent an economic loss of over $600/acre ($1,500/ha). Preliminary research has shown that BSM damage decreased relative feed quality (RFQ) of late-season bermudagrass hay by 7% on average. This decrease was attributed to lower total digestible nutrients (TDN) and slightly lower dry matter intake (DMI). Crude protein (CP) actually increased in the damaged bermudagrass, but this was a function of dilution of desirable carbohydrates. This would be similar to the phenomenon seen in weathered hay such that CP is actually higher in the outer edges of the hay bale where desirable carbohydrates have leached out of the bale while the nitrogen remains.
Growth Stages
Like other species in the Muscidae family, the adult stage of the BSM is a fly. The BSM fly is easier to find and identify than the larva or pupae because it occurs outside of the pseudostem and has distinct coloration (Figure 2). They have transparent wings, a gray thorax, and a yellow abdomen with at least one pair of black spots. Adult BSMs are about 1/8 inch (around 3.0 to 3.5 millimeters) in length. The females are typically larger than the males. The female abdomen is longer, more pointed, and curves under the fly’s body. In contrast, the male’s abdomen is shorter and more rounded. The proportion of female to males in a population varies from field to field and, perhaps, with the season. Data collected to date indicate that the females outnumber the males by an average of 4.6 to 1. However, this ratio has been observed to vary from 2:1 to 10:1.
The BSM female has the potential to lay a large number of eggs. Figure 3 shows the two ovaries of the female’s reproductive tract. Within each ovary, there are approximately 15 to 18 ovarioles. Each ovariole is capable of producing an oocyte (egg).
The larvae are white, cylindrical, and about 1/8 inch (3 millimeters) long when fully grown (Figure 4A). As they mature, their color gradually darkens to a tan or brown. The larvae also have mouth hooks that are barely visible to the naked eye. It is presumed that these mouth hooks enable the BSM to macerate the walls of the pseudostem. The metamorphosis of the BSM larvae into the adult fly occurs in a puparium, a rigid outer shell covering the pupae, that is orange to dark red and barrel-shaped, similar to that of other Atherigona species.
 Figure 2. The adult male (A) and female (B) bermudagrass stem maggot fly. Photo by Lisa Baxter.
Figure 2. The adult male (A) and female (B) bermudagrass stem maggot fly. Photo by Lisa Baxter.
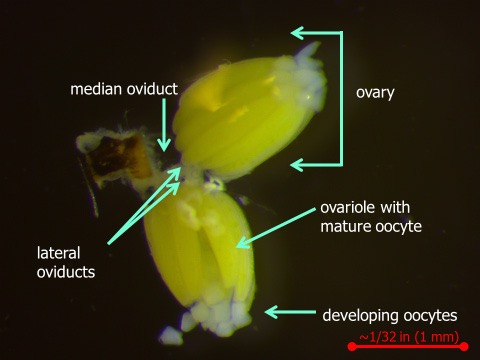 Figure 3. The reproductive tract of a female bermudagrass stem maggot fly. Photo by Lisa Baxter.
Figure 3. The reproductive tract of a female bermudagrass stem maggot fly. Photo by Lisa Baxter.
 Figure 4. Larvae (A) of the bermudagrass stem maggot are approximately 1/8 inch (3 millimeters) long. After feeding, the larva bores through the pseudostem leaving behind an exit hole (B). Photo by Lisa Baxter.
Figure 4. Larvae (A) of the bermudagrass stem maggot are approximately 1/8 inch (3 millimeters) long. After feeding, the larva bores through the pseudostem leaving behind an exit hole (B). Photo by Lisa Baxter.
Life Cycle
A better understanding of the bermudagrass stem maggot’s life cycle and biology has emerged as research on the timing of these biological phases continues. The life cycle begins with the BSM fly laying an egg on a bermudagrass leaf. The larva emerges approximately two to three days post-oviposition, or after eggs are laid, and slips or bores into the central whorl of the pseudostem. Once in the pseudostem, it begins to macerate the vascular tissue at the first node it encounters.
The lack of sap flow causes the top two to three leaves to become chlorotic, or yellowed due to insufficient clorophyll. Within one to two days after feeding begins, the first signs of damage are observed, and the affected leaves soon become completely chlorotic or necrotic (prematurely dying). Between the time when chlorosis is first observed and complete leaf senescence, or deterioration, the larva exits the stem (Figure 4B) and moves to the soil for pupation. The metamorphosis occurs in an orange-colored puparium over the course of 7 to 10 days and culminates with the emergence of the adult fly.
It has also been shown that when the pseudostem is cut (with a hay mower or grazed, for example), any viable larvae will exit the stem and move to the soil to pupate. As a result, adult flies begin to emerge in a sizeable flush 7 to 10 days after cutting. These findings have helped refine the timing for insecticide applications for suppressing adult populations during the first two to three weeks following a cutting (see the “Chemical Control” paragraph in the “Mitigation Strategies” section).
Adult flies live for approximately 18 to 20 days when kept in enclosures and provided sugar water. Actual adult life spans are estimated to be 14 to 21 days. Based on these observations, we believe the complete life cycle of the BSM to be three to four weeks long with multiple offspring being produced by the fly during its adulthood.
The degree to which the BSM overwinters in the Southeast remains unclear. We have observed that populations increase progressively from south to north, with high populations developing as early as mid-June in central Florida, early July in south Georgia, mid-July in central Georgia, and late July in north Georgia and points further north. This would indicate that overwintering success is, at a minimum, much better in more southern climes. Nonetheless, we have collected flies as early as February near Valdosta, Georgia, and mid-May near Athens, Georgia, so we presume they have at least some ability to overwinter at these latitudes.
Assessing BSM Populations
The orange, barrel-shaped puparium of the BSM may be found just under the soil surface when the insect is pupating. However, finding puparia in the soil has proven too challenging for a practical assessment of BSM populations. Consequently, no protocols have been developed to search for the pupae in a damaged field’s soil.
Finding the larva is only slightly less challenging, as it requires dissecting pseudostems as soon as they show the first signs of chlorosis in response to BSM damage. If the pseudostem shows extensive damage, then it is likely the larva has already left the pseudostem to pupate. Pseudostems may be carefully dissected using a sharp knife or razor blade, splitting the stem until the center of the shoot is revealed. Because of the small size of the larva, it is best to work over a solid, dark-colored surface so that the larva is not lost during the procedure.
Producers can use sticky traps or sweep nets to collect and identify the BSM fly in the field relatively easily (Figure 5). However, they tend to stay down in the forage canopy and rarely fly higher than 18 inches (0.5 meter) above the canopy. To date, sticky traps have only been useful in alerting one to the presence of the BSM because fly counts on sticky trap cards have not yet been observed to be correlated with fly populations. Sweep net estimates have been found to be relatively accurate predictors of actual fly populations in the field (Figure 6). Correlating fly populations to actual yield loss has proven much more challenging than simply catching and counting flies. The amount of damage is not merely a function of fly populations because a number of other factors also can influence yield loss. These include, but are not limited to, bermudagrass variety, timing of the infestation, number and proximity of bermudagrass fields near the field in question, timing of when those neighboring fields were last cut, amount of disease present in the crop, and the amount of fertilization added to the crop.



 Figure 5. Sticky traps (A) and sweep nets (B) can be used to catch flies. After dumping the catch from 10 sweeps with the net in a collapsible observation cage (C), one can estimate the number of insects collected (D).
Figure 5. Sticky traps (A) and sweep nets (B) can be used to catch flies. After dumping the catch from 10 sweeps with the net in a collapsible observation cage (C), one can estimate the number of insects collected (D).
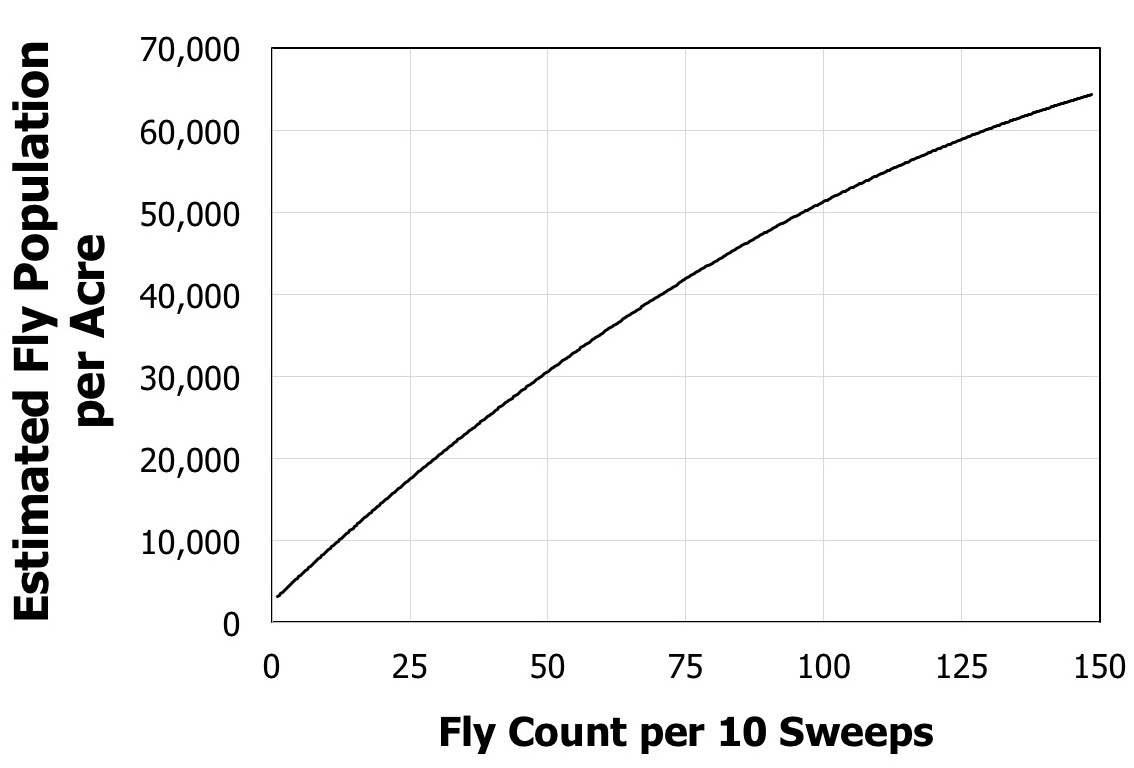 Figure 6. Estimated fly population per acre predicted from the number of flies counted per 10 sweeps with a sweep net.
Figure 6. Estimated fly population per acre predicted from the number of flies counted per 10 sweeps with a sweep net.
Varietal Differences
Research into damage by the BSM has shown that varieties that produce finer leaves or pseudostems and/or produce more pseudostems per square foot (or square meter) tend to be more susceptible to damage and yield loss. In general, the coarse-textured varieties of stargrass (Cynodon nlemfuensis Vanderyst) and hybrids of bermudagrass and stargrass (cv. ‘Tifton 85’, ‘Coastcross-I’, and ‘Coastcross-II’) are less susceptible to BSM damage. While these varieties have fewer tillers, proportionately fewer of those tillers are damaged. Cultivars with a higher number of shoots also tend to have a smaller shoot diameter, narrower leaves, and a lighter green color. These plant characteristics create a denser forage canopy, which seems to attract the BSM (Figure 7).
Consequently, some varieties of bermudagrass are more susceptible to damage by the BSM than are others. Table 1 reports the common yield loss observed in research trials conducted by the University of Georgia and the U.S. Department of Agriculture’s Agricultural Research Service (USDA-ARS) in Tifton, Georgia, as well as observations made by 海角官方首页 Extension agents and bermudagrass producers in Georgia. Though substantial yield loss can certainly be possible in the less susceptible varieties, performance is generally greater. Ongoing plant breeding efforts at the USDA-ARS in Tifton have shown progress in developing more varieties that are high yielding and high