For questions about this or other food science topics, please contact Extension Food Science at efs@uga.edu or call 706-542-2574.
Acid Foods v. Acidified Foods
An acidified food is a low-acid food to which acids (such as vinegar, lemon juice, citric acid, etc.) or acid foods (such as fruits or tomatoes) have been added to bring the equilibrium pH of the food to 4.6 or less, with equilibrium water activity greater than 0.85. Water activity in food can be thought of as the “free” water or water that’s not chemically bound to something.
A common example of an acidified food is a pickle—a low-acid, high-moisture cucumber with a natural pH greater than 4.6 and water activity greater than 0.85 is immersed in an acidic vinegar brine with spices to drop the pH of the cucumber to less than 4.6.
Acidified foods are considered shelf-stable because they do not need refrigeration while in their containers. The acids act as a preservative for the food, and pathogens are prevented from growing in acidified foods. Manufactured foods such as sodas, fruit juices, and salad dressings have a pH that falls under 4.6.
Why a Food’s pH Matters
The pH of a food is one of the factors that determines how well pathogens can survive and grow in it. The pH can be defined as the acidity of the product and is measured using a specialized instrument called a pH meter. It is measured on a 14-point scale, where pH of 7 is considered neutral, a pH greater than 7 is considered basic (also sometimes called alkaline), and a pH less than 7 is considered acidic.
Most foods fall in the acidic category (see Figure 1). Foods are further classified by whether their pH permits the growth of Clostridium botulinum. The C. botulinum pathogen can produce a harmful toxin when it is allowed to grow, but it can be maintained in its spore form, which does not pose a risk to human health. One of the ways to keep C. botulinum from being harmful to humans is to maintain an equilibrium pH in the food of 4.6 or lower.
In canned foods, the hermetic seal prevents oxygen from entering a container and creates an anaerobic (no-oxygen) environment, which is a prerequisite for C. botulinum to grow and produce its toxin. Controlling the pH of the food is the main way to keep C. botulinum in its sporulated form, so it is not able to produce its toxin.
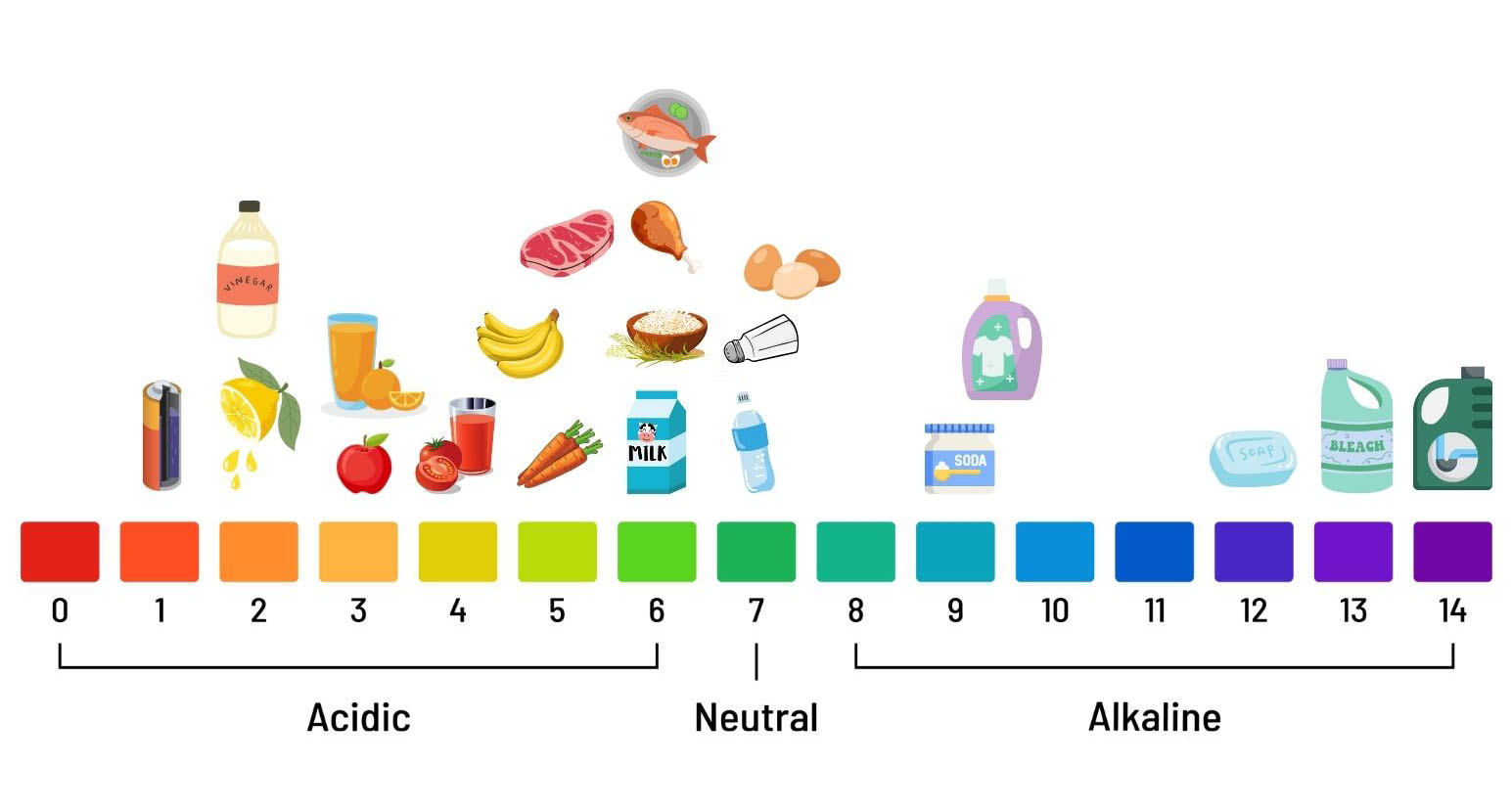
Figure 1. The pH Scale With Common Foods Represented. Most foods fall in the acidic category (pH 0–7). Fruits tend to have the lowest pH because of their acid content, followed by vegetables, which typically have pH > 4.6. Meats, seafood, and dairy are the closest to a neutral pH.
It's important to note that pH values will vary depending on factors such as ripeness, processing methods, and storage conditions.
| Category of food |
Associated pH range |
| Acid food |
Food with natural pH < 4.6 |
| Low-acid food |
Food with natural pH > 4.6 |
| Acidified food |
Low-acid food to which acids or acid foods have been added to reduce the equilibrium pH to 4.6 or less |
How to Acidify a Food
There are multiple ways to acidify a food product, including heating ingredients in an acidified aqueous solution, immersing blanched foods in an acid solution, direct acidification, and adding acid foods to low-acid foods. Each method is described in greater detail below.
Immersing Ingredients in an Acidified Aqueous Solution
This process is best suited for larger food particles. The food is added to a hot acid bath and heated. The acidification process, and how quickly the pH of the food drops to 4.6 or below, is dependent on time, temperature, type of acid, concentration of acid, and the size of the food pieces.
Heating or blanching prior to immersing can help acidify the product more quickly. If using the same acid bath for multiple batches, the pH of the bath must be checked and maintained since water can leach out from products and dilute the solution.
Direct Acidification
This process is best suited for liquid products that may contain small particles. Acid can be added directly to a batch of product in a mixing kettle. Heating can help acidify the small particles more quickly. Acid should be added until the target pH is reached.
Direct acidification can also be achieved by adding acid to individual containers, but this practice is not recommended because of the potential for skipping containers and the possibility of acid not reaching the entire product (in the case of a powdered or pelleted acidifier that may remain at the bottom of the container). If this method is used, the acidifier should be added to the container first, and then the food. Additionally, this method requires that the acid be mixed thoroughly with all of the food.
Adding Acid Foods to Low-Acid Foods
Just like acids, such as vinegar and citric acid, can be added to low-acid foods to acidify them, acid foods like tomatoes and fruit juices can also be added to low-acid foods as an acidification process. As with other methods, the foods must be mixed thoroughly, and the acidification process should allow for every food component to be acidified to a pH below 4.6. The ratio of acid foods to low-acid foods should be consistent to ensure adequate addition of acid.
Check the pH of Every Batch
Because the pH of a food will not be consistent between batches (see Figure 1), the pH should be measured every time to ensure the final pH of the food is below 4.6. More acid may need to be added to ensure the food reaches the proper pH.
Summary
Acidification is an effective method for food preservation and provides us with many commercially available shelf-stable foods. Acidification is just one way to preserve foods, and it is important to consider whether other factors have an impact on the safety and shelf-stability of the food. The other two 海角官方首页 Extension publications in this Acidified Foods series go into greater detail about the process steps and what food products are most suitable for HFH.
It is important to consult with a process authority when designing acidification processes for preserving foods. Process authorities may be found by contacting your local Extension office, through trade groups, or in the food industry.
Suggested Reading and References
Acidified Foods (Title 21), 44 C.F.R. § 16235 (1979).
Cornell Food Venture Center. (n.d.). Acid & acidified foods. Cornell Cooperative Extension.
Georgia Department of Agriculture. (n.d.). Food processing safety guidelines.
Johnson, L. (2021). Acidified foods. North Carolina State Extension.
Status and Revision History
In Review on May 01, 2025
Published on Jun 16, 2025